Better medical devices
Medical doctors and engineers developing medical devices best in collaboration as inter disciplinary team.
Medical device making
We manufacture in ISO class 6 clean room environment fulfilling the hurdle of ophthalmic operational devices...
Device R&D
We develop products with unique properties which are not available in the current market of devices...
Seemless production
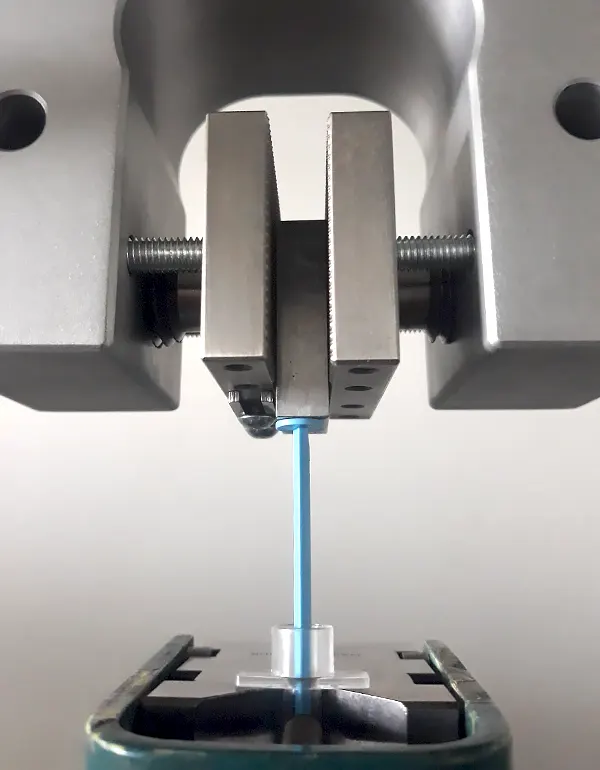
We understand, after many years of investigations, how to avoid stick / slip effects in barrel / piston combinations...
Meditech
Medical devices for the global market
Medical devices have become the lifeline for progress in our ever aging population around the globe. One big role in this progress is provided by secure and innovative medical devices made from engineering plastics.
We operate at both ends of the scale, both as a supplier of components and as a medical device marketer under EU Regulation 745/2017 (MDR). We focus on class II devices. Medical devices are an extension of our area of expertise, as the key is still material development, product design and mold design and manufacturing. The requirements are different, but challenge us to grow beyond the usual industrial boundaries.
We operate at both ends of the scale, both as a supplier of components and as a medical device marketer under EU Regulation 745/2017 (MDR). We focus on class II devices. Medical devices are an extension of our area of expertise, as the key is still material development, product design and mold design and manufacturing. The requirements are different, but challenge us to grow beyond the usual industrial boundaries.
Future
Device research & development
We develop products with unique features that are not available in the current device market. Most suggestions result from discussions with physicians.
We develop solutions that comply with DIN EN ISO 14971 and capture the core of the standard from start to finish. The core of this new risk management standard is to avoid risks for physicians or even for the patient in the frame. We develop all technical documentation details for the conformity process to get the CE marking from the notified body for the product in scope.
insight
Device R&D
according DIN EN ISO 13485 Chapter 7
Since you never stop learning, we also strive to meet all PMS (Post Market Surveillance) requirements in accordance with the MDR Regulation. A great help in all these activities is our in-house laboratory, which enables in-depth investigations over long periods of time and under a wide range of conditions to ensure reliable validation and verification results.
The graph shown here shows our results for the absence of particles < 1 micron / < 2 micron / < 5 micron as a comparison between the new device and current market standards.
Innovation
PFAS-free medical solutions
Experience the future of medical care with PFAS-free options. Our pioneering products offer superior safety and a commitment to a sustainable future for our planet.
Pioneering PFAS-free solutions! We’re proud to introduce the first-ever medical skin applicators with entirely PFAS-free adhesives. Unlike conventional options, our product ensures all materials – pads, adhesives, liners, and cardboard – are free of harmful PFAS.
Innovation
Medical device making
We manufacture in ISO class 6 clean room environment fulfilling the hurdle of ophthalmic operational devices for both particles and bioburden. All Molds and all equipment is made internally and we fulfill all requirements of DIN EN ISO 13485:2016 accordingly.
All Device making is in accordance to the current MDPG and we are yearly Audited by multiple official organisations. Among our own products, we offer to make devices or components for devices for others. As we consider this a good addition to gain more insight into this challenging market- we offer secure and safe making and handling in the bioburden controlled environment. For all sterilisation tasks we reach out to our partners from HA2 Medizintechnik.
We focus our efforts on medical devices that we market in the form of sterile syringes for ophthalmology, specifically for use with humanized antibodies targeting AMD (macular degeneration) of the human eye.
We are supplying empty, 100% silicone-free syringes that contain no residue other than the bore of the needle. Our validation results for the in-use syringes have shown that 30 times fewer particles of 1 micron size were measured in the transition fluids compared to devices in use today.
The second medical device currently in the approval phase according to MDR is the so-called „PRP hourglass device“.
This device separates the three blood fractions of human whole blood and enables safe handling during the entire preparation process of platelets intended for injection into the shoulder, knee or other areas of the human body to promote healing and reconstruction of lost or worn structures.
Experience
Fields of knowhow
In the 8 years we have spent in research, development and manufacturing of medical devices, we have been able to gather the following know-how: After many years of research, we know how to avoid stick/slip effects in cylinder/piston combinations without lubricants (e.g. syringe applications).
All the principles and techniques developed enable devices with best-in-class particle analytics (0.9 micron – 50 micron size). The team has learned how to use TPE materials with bioinert properties (according to DIN EN ISO 10993-1 and Pharmacopoeia) so that they can be stored for long periods of time without the so-called disadvantages of compression set and thus reducing the risk of dosing errors when administering the therapeutic dose. One of the things we have learned is how to individualise devices (i.e. unit 1) to enable tracking and tracing down the pharmaceutical line to the patient. This technology has been developed and can be applied in a GMP 0 environment. Recently, we have learned how to deal with extreme G-forces in medical devices that need to be biocompatible and still withstand the G-forces that occur during centrifugation.